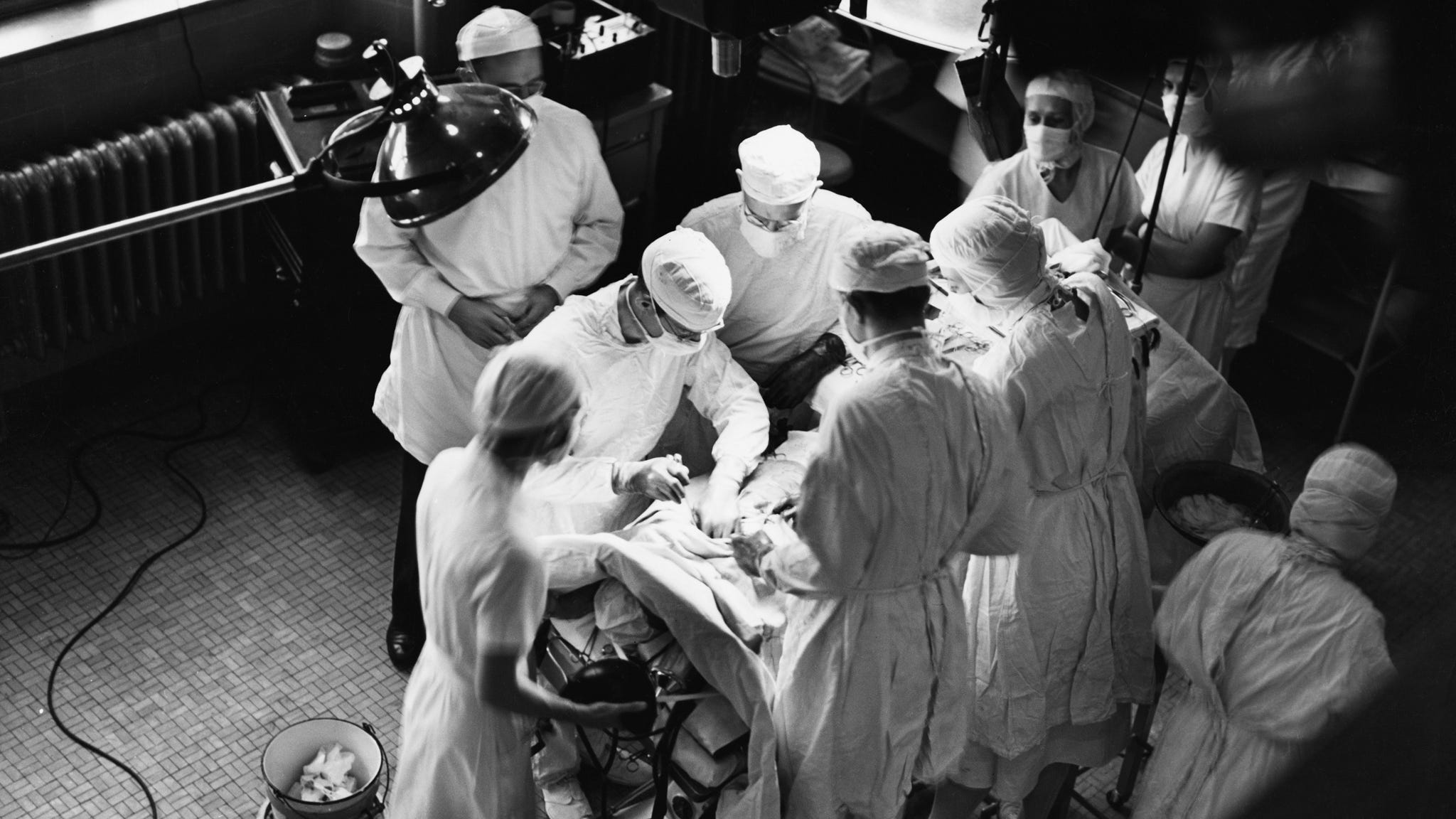
The Underdog Doctors Who Saved America’s “Blue Babies”
In 1945, a chain-smoking surgeon, a deaf female doctor, and a self-taught African-American lab tech developed a risky procedure that revolutionized medicine.
Illustrations by Vinnie Neuberg
One afternoon in November 1944, the chief of surgery at Johns Hopkins Hospital in Baltimore, Alfred Blalock, sat in his office deep in thought. As usual, he had a cigarette on the go: even after losing two years of his early career to tuberculosis, he had never quite managed to give up his 40-a-day habit. With his neatly combed hair, immaculate chalk-stripe suit and donnish glasses, he might easily have been mistaken for a prosperous lawyer, but at the age of 45, he was already known as one of America’s foremost clinical researchers. A few years earlier he had revolutionized the treatment of circulatory shock, a life-threatening condition in which blood loss makes it difficult for the heart to pump enough fluid to the body. Shock was one of the biggest killers in wartime, frequently the consequence of injury by shrapnel or explosives. Blalock’s experiments led to the routine use of blood-plasma transfusions to treat those with severe wounds, a measure wh…
Keep reading with a 7-day free trial
Subscribe to Narratively to keep reading this post and get 7 days of free access to the full post archives.


